
***********************************************************
WYFF4.com
Fungal, bacterial growth found in steroid
Tenn. pharmacy under investigation for links to reported infections
UPDATED 11:16 AM EDT Jun 10, 2013
The
U.S. Food and Drug Administration has identified bacterial and fungal
growth in unopened vials of a steroid injection from a Tennessee
pharmacy under investigation for links to reported infections.
Main Street Family Pharmacy of Newbern, Tenn., issued a voluntary recall nationwide of all lots of sterile products that the pharmacy compounds on May 28. Products with a use by date on or before Nov. 20, 2013, are subject to the recall.
The steroid in question is preservative-free methylprednisolone acetate. Samples from two separate batches were found to have microbial growth in them, the FDA said.
"At this point in FDA's investigation, the sterility of all sterile products produced by Main Street is of significant concern and the products should not be used," the FDA said.
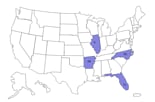
As of Thursday, the Centers for Disease Control and Prevention reported 24 cases of infection from four states -- Arkansas, Florida, Illinois and North Carolina. Most of these people developed skin and soft tissue infections after receiving the intramuscular injection of the steroid.
Main Street Family Pharmacy of Newbern, Tenn., issued a voluntary recall nationwide of all lots of sterile products that the pharmacy compounds on May 28. Products with a use by date on or before Nov. 20, 2013, are subject to the recall.
The steroid in question is preservative-free methylprednisolone acetate. Samples from two separate batches were found to have microbial growth in them, the FDA said.
"At this point in FDA's investigation, the sterility of all sterile products produced by Main Street is of significant concern and the products should not be used," the FDA said.
As of Thursday, the Centers for Disease Control and Prevention reported 24 cases of infection from four states -- Arkansas, Florida, Illinois and North Carolina. Most of these people developed skin and soft tissue infections after receiving the intramuscular injection of the steroid.
*******************************************************
CDC
Multistate Investigation of Suspected Infections Following Steroid Injections
Posted June 13, 2013 3 PM ET
At a Glance:
- Status: On-going Investigation
- Reported Cases: 26
- States reporting cases: 4
- Recall: Yes

Distribution maps
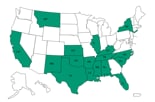
States reporting cases *
States that received recalled MPA
This website will be updated on Thursdays by 3pm ET.
Initial Announcement
May 30, 2013
Main Street Family Pharmacy in Tennessee: FDA Alerts Health Care Providers of Adverse Reactions Associated with Steroid InjectionsState, Federal Health Officials Investigate Reports of Adverse Events among Patients Receiving Methylprednisolone Acetate Injections
Summary of Investigation
This information is preliminary and will be updated as additional details become available.
June 13, 2013
CDC and FDA have identified the bacteria and fungi cultured from unopened vials of preservative-free MPA from Main Street Family Pharmacy (MSFP) in Newbern, TN. These findingsIn addition to the findings above, 4 of the 26 cases meeting the CDC case definition* have had bacteria or fungi detected from wounds: 2 patients had Enterobacter cloacae and Klebsiella pneumoniae, 1 had mixed bacterial culture not otherwise identified, and 1 had fungus highly suggestive of an Aspergillus sp., although further studies are needed for confirmation. Although bacteria and fungi have been isolated from unopened vials of MPA from MSFP, it is not possible to determine which infections are due to this contamination event versus other factors including improper handling and/or administration of medications at the injection facility.
Clinicians are reminded that they should 1) use individual containers of compounded or preservative-free medicine for a single-patient only, and 2) promptly report to MedWatch any infection that might be related to a medication or medical device, even absent a recognized outbreak, as these reports can allow for early detection of a possible contamination event.
Note: The next CDC web update is scheduled for Thursday, June 20.
***************************************************
Related articles
- Fungus found steroid shots (cnn.com)
- Fungus found steroid shots (cnn.com)
- FDA finds fungus, bacteria in recalled pain shots (lunaticoutpost.com)
- FDA finds fungus, bacteria in recalled pain shots (vitals.nbcnews.com)
- Fungal, bacterial growth found in steroid injections (wtkr.com)
- FDA finds fungus in drugs from Tennessee pharmacy (miamiherald.com)
- FDA Finds Fungus in Drugs from Tennessee Pharmacy (sci-tech-today.com)
- Multistate outbreak linked to Main Street Family Pharmacy in Tennessee stands at 20 cases, Florida reports 13 (theglobaldispatch.com)

No comments:
Post a Comment
Hello and thank you for visiting my blog. Please share your thoughts and leave a comment :)